
Chinese Journal OF Rice Science ›› 2016, Vol. 30 ›› Issue (5): 447-457.DOI: 10.16819/j.1001-7216.2016.6048
• Orginal Article • Next Articles
Peng-yi PAN, Jian-ping ZHU, Yun-long WANG, Yuan-yuan HAO, Yue CAI, Wen-wei ZHANG, Ling JIANG, Yi-hua WANG, Jian-min WAN*( )
)
Received:2016-03-19
Revised:2016-04-28
Online:2016-09-10
Published:2016-09-10
Contact:
Jian-min WAN
潘鹏屹, 朱建平, 王云龙, 郝媛媛, 蔡跃, 张文伟, 江玲, 王益华, 万建民*( )
)
通讯作者:
万建民
基金资助:CLC Number:
Peng-yi PAN, Jian-ping ZHU, Yun-long WANG, Yuan-yuan HAO, Yue CAI, Wen-wei ZHANG, Ling JIANG, Yi-hua WANG, Jian-min WAN. Phenotyping and Gene Cloning of a Floury Endosperm Mutant ws in Rice[J]. Chinese Journal OF Rice Science, 2016, 30(5): 447-457.
潘鹏屹, 朱建平, 王云龙, 郝媛媛, 蔡跃, 张文伟, 江玲, 王益华, 万建民. 水稻粉质胚乳突变体ws的表型分析及基因克隆[J]. 中国水稻科学, 2016, 30(5): 447-457.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2016.6048
| 标记 Marker | 正向引物Forward (5'→3') | 反向引物Reverse (5'→3') |
|---|---|---|
| HY8-19 | TTTGTTGCTTTTCTGATTC | ATGATAAAGCGATAAACCA |
| WQ8-28 | GAGACGGACGGGTGTTGA | CAATGACATCCCAGCGTA |
| WZ8-17 | TAAATCATGGTGGTGGGC | ACCGTCGTCTAGCAAGGAG |
| WZ8-3 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| HY8-24 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
Table 1 Markers for fine mapping of WS.
| 标记 Marker | 正向引物Forward (5'→3') | 反向引物Reverse (5'→3') |
|---|---|---|
| HY8-19 | TTTGTTGCTTTTCTGATTC | ATGATAAAGCGATAAACCA |
| WQ8-28 | GAGACGGACGGGTGTTGA | CAATGACATCCCAGCGTA |
| WZ8-17 | TAAATCATGGTGGTGGGC | ACCGTCGTCTAGCAAGGAG |
| WZ8-3 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| HY8-24 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| 基因 Gene | 正向引物 Forward (5'→3') | 反向引物 Reverse (5'→3') |
|---|---|---|
| AGPL1 | CATCAAGGACGGGAAGGTCA | ACTTCACTCGGGGCAGCTTA |
| AGPL2 | CTGAGGAAGAGGTGCTTTGG | TCTTTCGGGAGGATTGTGTC |
| AGPS1 | AGAATGCTCGTATTGGAGAAAATG | GGCAGCATGGAATAAACCAC |
| AGPS2a | ACTCCAAGAGCTCGCAGACC | GCCTGTAGTTGGCACCCAGA |
| AGPS2b | AACAATCGAAGCGCGAGAAA | GCCTGTAGTTGGCACCCAGA |
| UGPase1 | CCATCACCGCCAAGTCA | GACCGTTGATGTCCTTGTTCT |
| Actin | CCCTCCTGAAAGGAAGTACAGTGT | GTCCGAAGAATTAGAAGCATTTCC |
Table 2 Primers used in real-time RT-PCR.
| 基因 Gene | 正向引物 Forward (5'→3') | 反向引物 Reverse (5'→3') |
|---|---|---|
| AGPL1 | CATCAAGGACGGGAAGGTCA | ACTTCACTCGGGGCAGCTTA |
| AGPL2 | CTGAGGAAGAGGTGCTTTGG | TCTTTCGGGAGGATTGTGTC |
| AGPS1 | AGAATGCTCGTATTGGAGAAAATG | GGCAGCATGGAATAAACCAC |
| AGPS2a | ACTCCAAGAGCTCGCAGACC | GCCTGTAGTTGGCACCCAGA |
| AGPS2b | AACAATCGAAGCGCGAGAAA | GCCTGTAGTTGGCACCCAGA |
| UGPase1 | CCATCACCGCCAAGTCA | GACCGTTGATGTCCTTGTTCT |
| Actin | CCCTCCTGAAAGGAAGTACAGTGT | GTCCGAAGAATTAGAAGCATTTCC |

Fig. 1. Phenotype comparison of the wild-type and ws mutant. A, Comparison of wild-type and ws mutant seeds, bar = 3 mm; B, Seed cross-sections of wild-type and ws mutant, bar = 1 mm; C, Comparison of wild-type and ws mutant plants, bar = 20 cm. DJY, Dianjingyou 1(wild type).
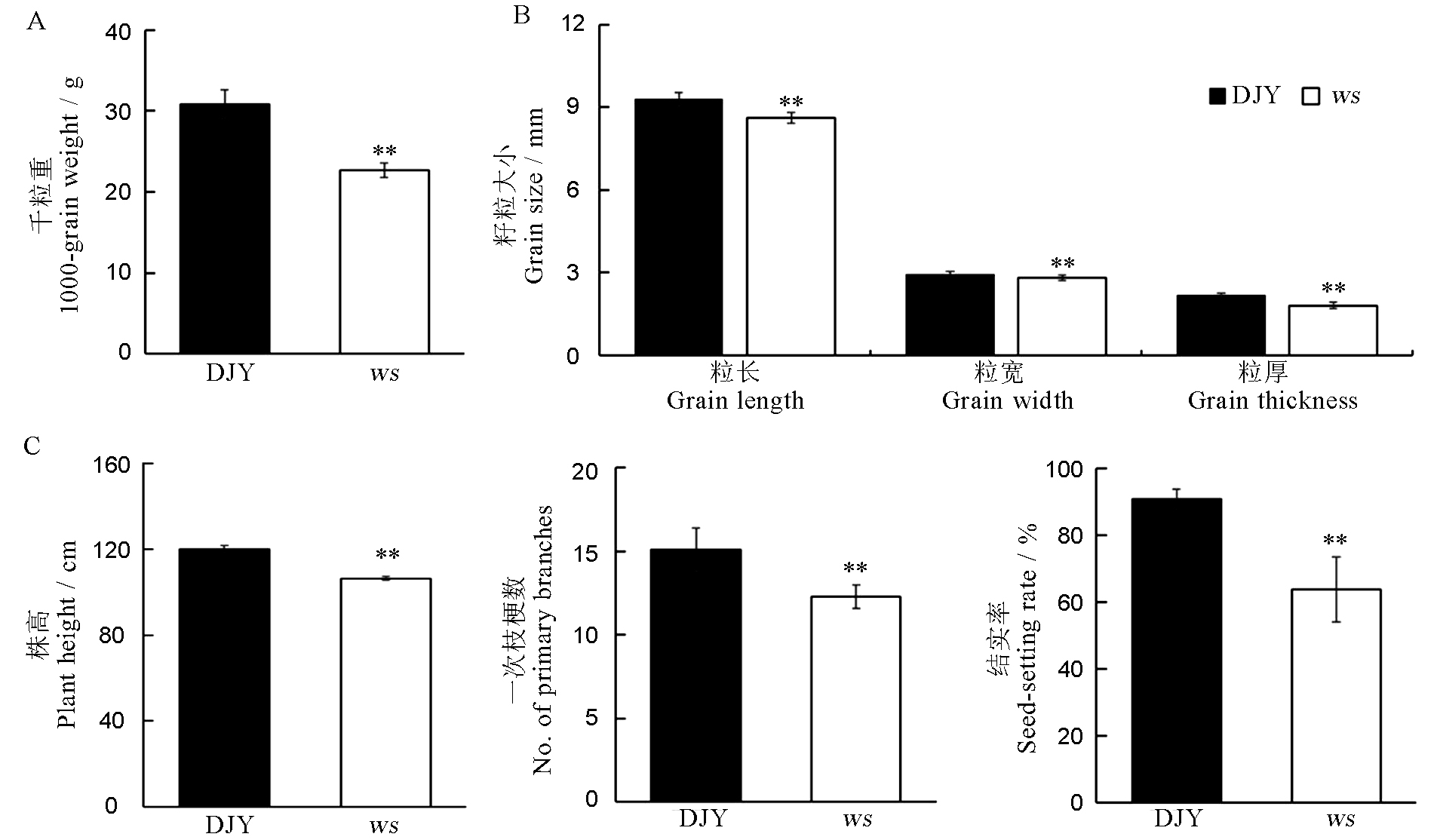
Fig. 2. Comparison of 1000-grain weight, seed and major agronomic traits of wild-type and ws mutant. Values are mean ± SD (n = 10, except for 1000-grain weight, n = 3); t-test, ** P < 0.01. DJY, Dianjingyou 1(wild type).
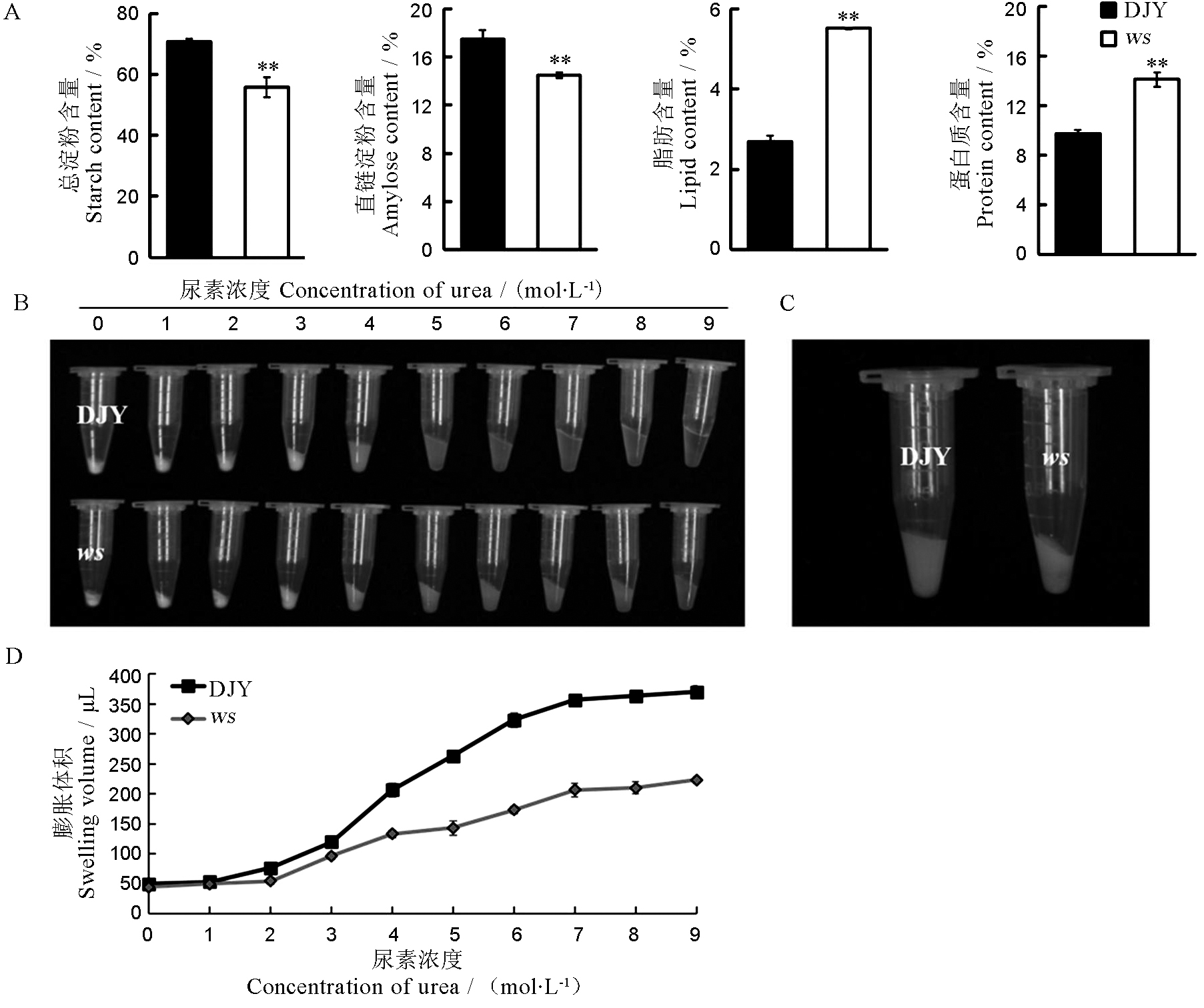
Fig. 3. Physicochemical characteristics of wild-type and ws mature seeds. A, Comparison of chemical composition of wild-type and ws seeds, values are mean ± SD, n = 3; t-test, ** P < 0.01; B, Gelatinization properties of wild type and ws mutant seeds; C, Significant difference was observed at the urea concentration of 4 mol/L urea; D, The swollen volume of wild-type and ws starch in urea solutions of various concentrations (n = 3). DJY, Dianjingyou 1(wild type).
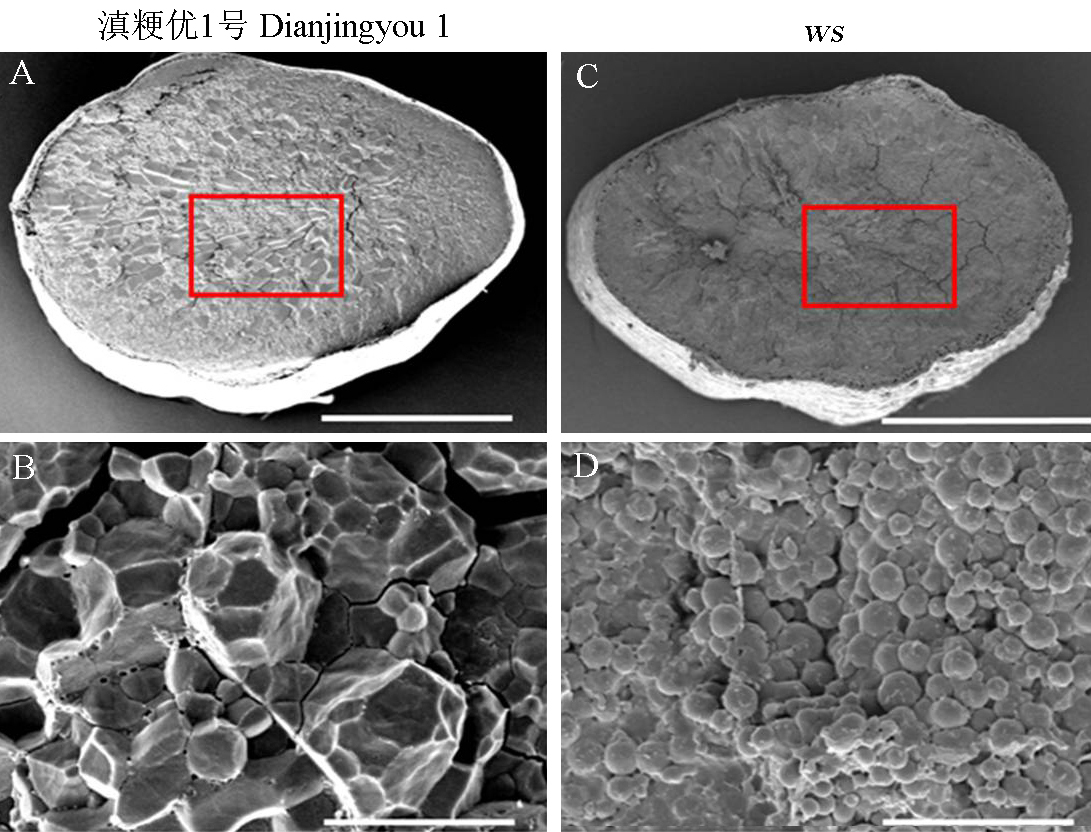
Fig. 4. Scanning electron microscopy observation of mature seeds of wild type and ws mutant. A, B, Endosperm of wild-type; C, D, Endosperm of ws mutant; A, C, Endosperm cross-section, Bars = 1 mm; B, D, Partial enlarged drawing picture, Bars = 10 μm.

Fig. 5. Semi-thin sections of wild type and ws mutant seeds. A, B, Peripheral part (A) and central part (B) of wild-type endosperm cells at 9 DAF (days after flowering); C, Central part of wild-type endosperm cells at 12 DAF; D, E, Peripheral part (D) and central part (E) of ws endosperm cells at 9 DAF; F, Central part of ws endosperm cells at 12 DAF. Bars = 100 μm in A and D, 50 μm in B, C, E, F.
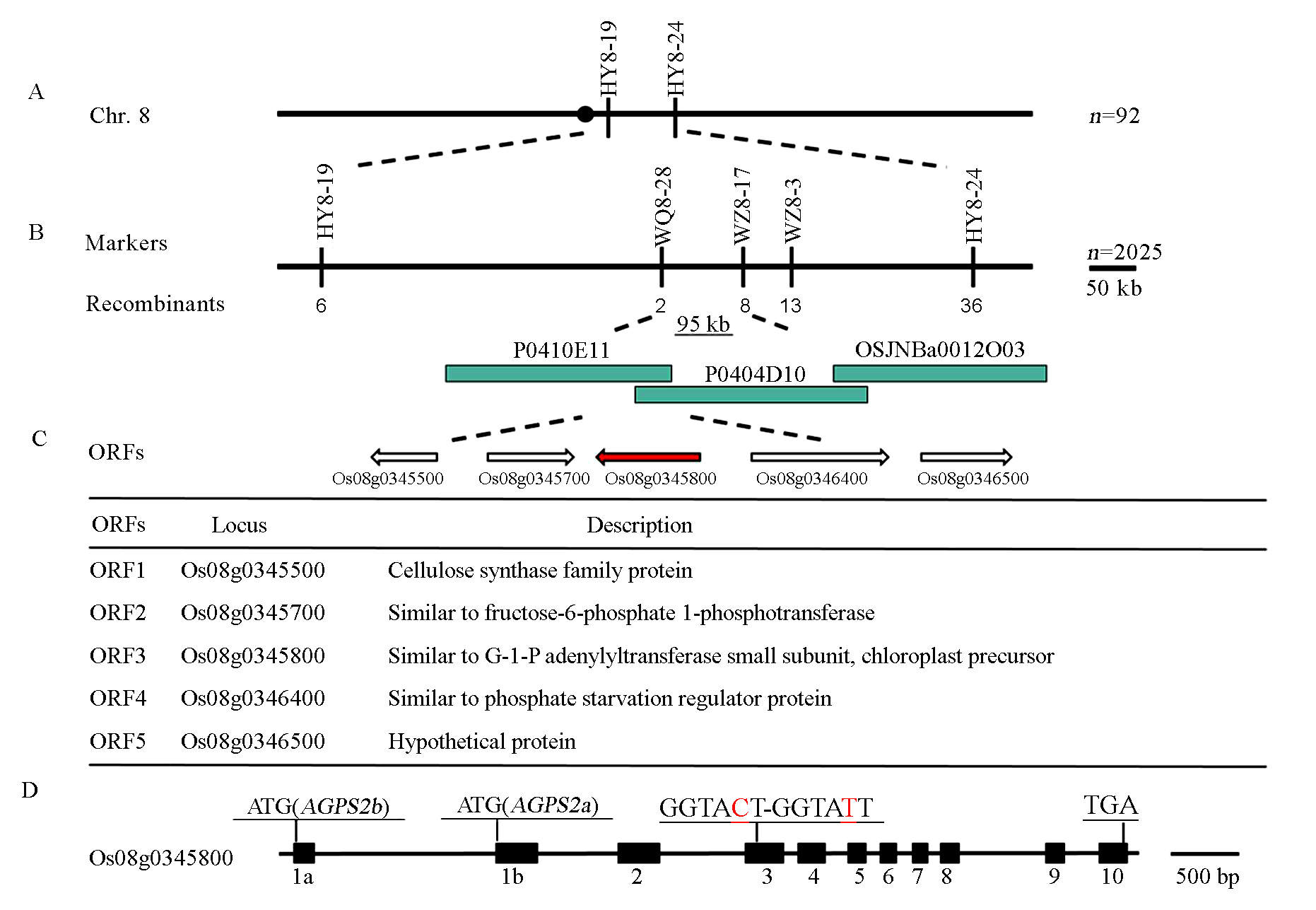
Fig. 6. Fine-mapping of WS gene. A, WS was linked with markers HY8-19 and HY8-24; B, WS was located in a 95 kb region based on 2025 individuals; C, Candidate genes for WS; D, ws displayed a single nucleotide substitution in AGPS2 (in red),AGPS2 is composed of 10 exons (filled box) and 9 introns, the alternative use of exon 1a and 1b generates AGPS2a and AGPS2b transcripts, respectively.
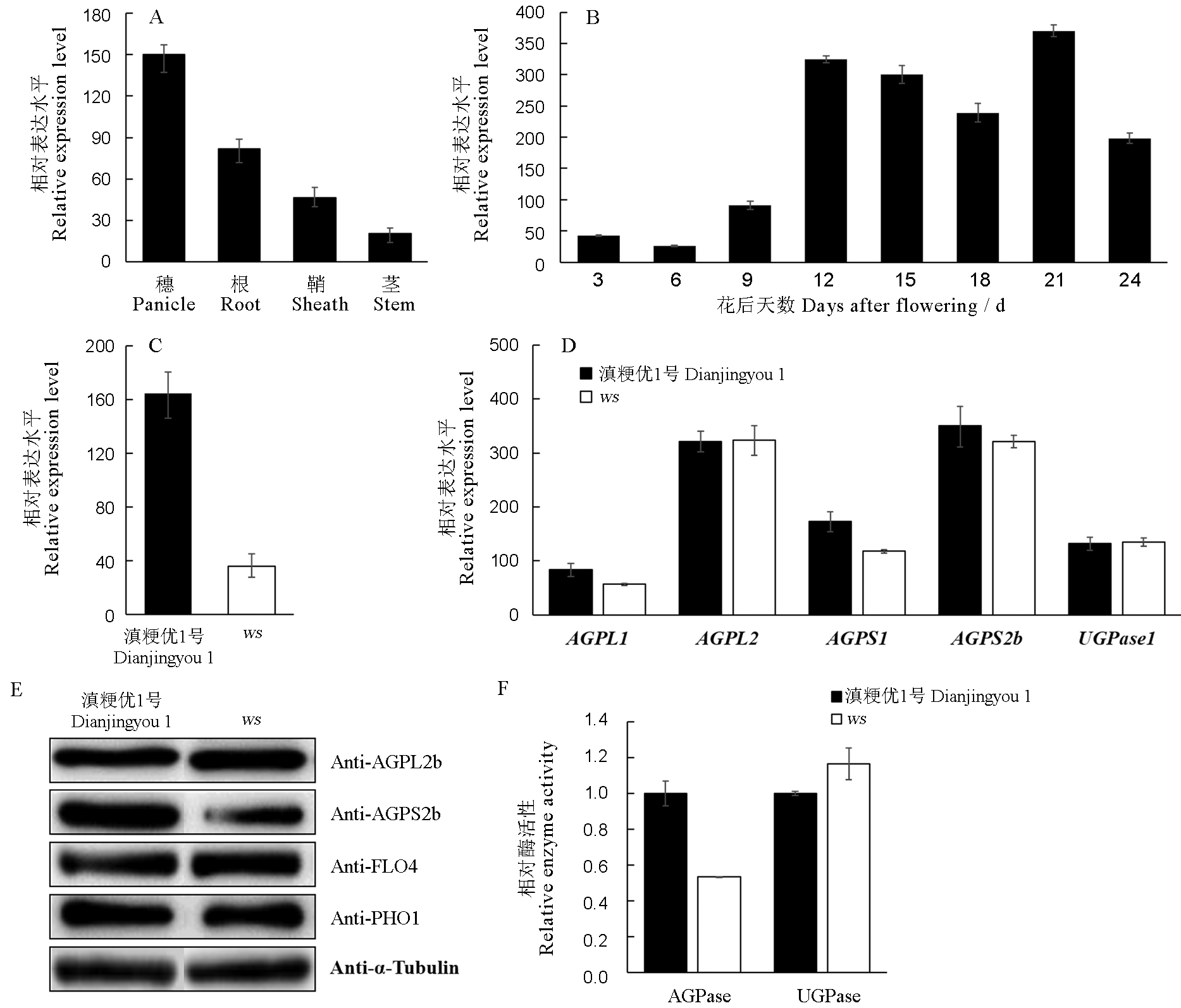
Fig. 7. Expression of related genes and enzyme activity in wild type and ws mutant. A, Real-time RT-PCR analysis of the expression of AGPS2b in different organs in wild type; B, Real-time RT-PCR analysis of the expression of AGPS2b at different developing stages of endosperm in wild type; C, Real-time RT-PCR analysis of the expression of AGPS2a in leaves of the wild type and ws mutant seedlings at 7 days after germination; D, Real-time RT-PCR analysis of the expression of genes encoding AGPase in wild type and ws mutant endosperm; E, Immunoblot analysis of starch biosynthesis related proteins in wild type and ws mutant endosperm; F, AGPase and UGPase activities of wild-type and ws developing endosperm. For A, B, C, D and F, error bars show SD (n = 3).
| [1] | Buléon A, Colonna P, Planchot V, et al.Starch granules: Structure and biosynthesis.Int J Biol Macr, 1998, 23(2): 85-112. |
| [2] | Hirose T, Terao T.A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.) .Planta, 2004, 220(1): 9-16. |
| [3] | Ohdan T, Francisco P B, Sawada T, et al.Expression profiling of genes involved in starch synthesis in sink and source organs of rice.J Exp Bot, 2005, 56(422): 3229-3244. |
| [4] | Tetlow I J, Morell M K, Emes M J.Recent developments in understanding the regulation of starch metabolism in higher plants.J Exp Bot, 2004, 55(406): 2131-2145. |
| [5] | Fujita N, Satoh R, Hayashi A, et al.Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIa.J Exp Bot, 2011, 62(14): 4819-4831. |
| [6] | Hanashiro I, Itoh K, Kuratomi Y, et al.Granule-bound starch synthase I is responsible for biosynthesis of extra-long unit chains of amylopectin in rice.Plant & Cell Physiol, 2008, 49(6): 925-933. |
| [7] | Liu L, Ma X, Liu S, et al.Identification and characterization of a novel Waxy allele from a Yunnan rice landrace.Plant Mol Biol, 2009, 71(6): 609-626. |
| [8] | Nishi A, Nakamura Y, Tanaka N, et al.Biochemical and genetic analysis of the effects of Amylose-Extender mutation in rice endosperm.Plant Physiol, 2001, 127(2): 459-472. |
| [9] | Satoh H, Nishi A, Yamashita K, et al.Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm.Plant Physiol, 2003, 133(3): 1111-1121. |
| [10] | Kubo A, Fujita N, Harada K, et al.The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm.Plant Physiol, 1999, 121(2): 399-410. |
| [11] | Fujita N, Toyosawa Y Y, Higuchi T, et al.Characterization of pullulanase (PUL)-deficient mutants of rice (Oryza sativa L.) and the function of PUL on starch biosynthesis in the developing rice endosperm.J Nano Res, 2008, 16(1): 43-48. |
| [12] | Preiss J.Bacterial glycogen synthesis and its regulation.Ann Rev Microbiol, 1984, 38(1):419-458. |
| [13] | Villand P, Olsen O A, Kleczkowski L A.Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana.Plant Mol Biol, 1993, 23(6): 1279-1284. |
| [14] | Okita T W, Nakata P A, Anderson J M, et al.The subunit structure of potato tuber ADPglucose pyrophosphorylase.Plant Physiol, 1990, 93(2): 785-790. |
| [15] | Takashi A, Kouichi M, Tatsuhito F.Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA.Plant & Cell Physiol, 2005, 46(6): 937-946. |
| [16] | Binquan H, Hennen-Bierwagen T A, Myers A M. Functions of multiple genes encoding ADP-glucose pyrophosphorylase subunits in maize endosperm, embryo, and leaf.Plant Physiol, 2014, 164(2): 596-611. |
| [17] | Sikka V K, Choi S B, Kavakli I H, et al.Subcellular compartmentation and allosteric regulation of the rice endosperm ADP-glucose pyrophosphorylase.Plant Sci, 2001, 161(3):461-468. |
| [18] | Tetlow I J, Davies E J, Vardy K A, et al.Subcellular localization of ADP-glucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform.J Exp Bot, 2003, 54(383):715-725. |
| [19] | Kawagoe Y, Kubo A, Satoh H, et al.Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm.Plant J, 2005, 42(2): 164-174. |
| [20] | Lee S K, Hwang S K, Han M, et al.Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.).Plant Mol Biol, 2007, 65(4): 531-546. |
| [21] | Johnson P E, Patron N J, Bottrill A R, et al.A low-starch barley mutant, risø 16, lacking the cytosolic small subunit of ADP-glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit.Plant Physiol, 2003, 131(2):81-99. |
| [22] | Sakulsingharoj C, Choi S, Hwang S, et al.Engineering starch biosynthesis for increasing rice seed weight: The role of the cytoplasmic ADP-glucose pyrophosphorylase.Plant Sci, 2004, 167(6):1323-1333. |
| [23] | Curtis L H, Brandon F, James B, et al.A shrunken-2 transgene increases maize yield by acting in maternal tissues to increase the frequency of seed development.Plant Cell, 2012, 24(6):2352-2363. |
| [24] | Satoh H, Omura T.New endosperm mutations induced by chemical mutagens in rice Oryza sativa L.Jpn J Breeding, 1981, 31(3):316-326. |
| [25] | She K C, Kusano H, Koizumi K, et al.A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality.Plant Cell, 2010, 22(10): 3280-3294. |
| [26] | Nishio T, Iida S.Mutants having a low content of 16-kDa allergenic protein in rice (Oryza sativa L.).Theor Appl Genet, 1993, 86(2-3): 317-321. |
| [27] | Kang H G, Park S, Matsuoka M, et al.White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB).Plant J, 2005, 42(6): 901-911. |
| [28] | Ryoo N, Yu C, Park C S, et al.Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.).Plant Cell Rep, 2007, 26(7):1083-1095. |
| [29] | Peng C, Wang Y, Liu F, et al.FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm.Plant J, 2014, 77(6): 917-930. |
| [30] | Zhang L, Ren Y, Lu B, et al.FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice.J Exp Bot, 2015, 67(3):633-647. |
| [31] | Wu K S, Tanksley S D.Abundance, polymorphism and genetic mapping of microsatellites in rice.Mol General Genet, 1993, 241(1-2): 225-235. |
| [32] | 李永庚, 于振文, 姜东, 等. 冬小麦旗叶蔗糖和籽粒淀粉合成动态及与其有关的酶活性的研究. 作物学报, 2001, 27(5): 658-664. |
| Li Y G, Yu Z, Jiang D, et al.Studies on the dynamic changes of the synthesis of sucrose in the flag leaf and starch in the grain and related enzymes of high-yield wheat.Acta Agron Sin, 2001, 27(5): 658-664. (in Chinese with English abstract) | |
| [33] | Denyer K, Dunlap F, ThorbjoRnsen T, et al. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial.Plant Physiol, 1996, 112(2): 779-785. |
| [34] | Thorbjornsen T, Villand P, Denyer K, et al.Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm.Plant J, 1996, 10(2): 243-250 |
| [35] | Hannah L C, Shaw J R, Giroux M J, et al.Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase.Plant Physiol, 2001, 127(1): 173-183. |
| [36] | Sikka V K, Choi S B, Kavakli I H, et al.Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase.Plant Sci, 2001, 161(3): 461-468. |
| [37] | Seon-Kap H, Yasuko N, Dongwook K, et al.Direct appraisal of the potato tuber ADP-glucose pyrophosphorylase large subunit in enzyme function by study of a novel mutant form.J Biol Chem, 2008, 283(11): 6640-6647. |
| [38] | Laughlin M J, Chantler S E, Okita T W.N- and C-terminal peptide sequences are essential for enzyme assembly, allosteric, and/or catalytic properties of ADP-glucose pyrophosphorylase.Plant J, 1998, 14(2): 159-168. |
| [39] | Pedro C, Ballicora M A, Angel M, et al.The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme.J Biol Chem, 2003, 278(31): 28508-28515. |
| [40] | Petreikov M, Eisenstein M, Yeselson Y, et al.Characterization of the AGPase large subunit isoforms from tomato indicates that the recombinant L3 subunit is active as a monomer.Biochem J, 2010, 428(2): 201-212. |
| [41] | Ventriglia T, Kuhn M L, Ruiz M T, et al.Two Arabidopsis ADP-glucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic.Plant Physiol, 2008, 148(1): 65-76. |
| [42] | Aytug T, Joe K, Yasuharu I, et al.The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme.Plant Cell Physiol, 2014, 55(6): 1169-1183. |
| [1] | HOU Xiaoqin, WANG Ying, YU Bei, FU Weimeng, FENG Baohua, SHEN Yichao, XIE Hangjun, WANG Huanran, XU Yongqiang, WU Zhihai, WANG Jianjun, TAO Longxing, FU Guanfu. Mechanisms Behind the Role of Potassium Fulvic Acid in Enhancing Salt Tolerance in Rice Seedlings [J]. Chinese Journal OF Rice Science, 2024, 38(4): 409-421. |
| [2] | ZHOU Tian, WU Shaohua, KANG Jianhong, WU Hongliang, YANG Shenglong, WANG Xingqiang, LI Yu, HUANG Yufeng. Effects of Planting Patterns on Starch Content and Activities of Key Starch Enzymes in Rice Grains [J]. Chinese Journal OF Rice Science, 2024, 38(3): 303-315. |
| [3] | WU Ziniu, HE Limei, XIONG Ying, CHEN Kairui, YANG Zhiyuan, SUN Yongjian, LÜ Xu, MA Jun. Effect of Nitrogen Fertilizer Topdressing for Panicle Differentiation on Grain Filling of Hybrid indica Rice and Its Relationship with the Activities of Key Enzymes for Starch Synthesis [J]. Chinese Journal OF Rice Science, 2024, 38(1): 48-56. |
| [4] | YONG Mingling, YE Miao, ZHANG Yu, TAO Yu, NI Chuan, KANG Yuying, ZHANG Zujian. Rice Starch Structure and Physicochemical Properties of Good Taste japonica Rice Varieties and Their Regulations by Nitrogen [J]. Chinese Journal OF Rice Science, 2024, 38(1): 57-71. |
| [5] | CHEN Liming, YANG Taotao, XIONG Ruoyu, TAN Xueming, HUANG Shang, ZENG Yongjun, PAN Xiaohua, SHI Qinghua, ZHANG Jun, ZENG Yanhua. Effect of Free-air Temperature Increasing on Activities of Enzymes Involved in Starch Synthesis and Accumulation of Double-cropping indica Rice [J]. Chinese Journal OF Rice Science, 2023, 37(2): 166-177. |
| [6] | CHEN Hongyang, JIA Yan, ZHAO Hongwei, QU Zhaojun, WANG Xinpeng, DUAN Yuyang, YANG Rui, BAI Xu, WANG Changcheng. Effects of Low Temperature Stress During Grain Filling on Starch Formation and Accumulation of Superior and Inferior Grains in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(5): 487-504. |
| [7] | LI Xia, JIANG Yanjie, TAO Yajun, LI Wenqi, WANG Fangquan, CHEN Zhihui, XU Yang, WANG Jun, FAN Fangjun, ZHU Jianping, Sreenivasulu NESE, YANG Jie. Research Progress of Rice with Low Glycemic Index [J]. Chinese Journal OF Rice Science, 2022, 36(4): 336-347. |
| [8] | HUANG Qina, JIANG Su, WANG Limin, ZHANG Yan, YU Linfei, LI Chunfu, DING Liqun, SHAO Guosheng. Effects of Moisture Content on Root Vigor and the Expression of Aquaporin-related Genes in Rice Seedlings Under Low Temperature Stress [J]. Chinese Journal OF Rice Science, 2022, 36(4): 367-376. |
| [9] | SHEN Hong, YAO Dongping, WU Jun, LUO Qiuhong, WU Zhipeng, LEI Dongyang, DENG Qiyun, BAI Bin. Effects of High Temperature in Various Phases of Grain Filling on Rice Starch Physicochemical Properties [J]. Chinese Journal OF Rice Science, 2022, 36(4): 377-387. |
| [10] | YAO Shu, ZHANG Yadong, LU Kai, WANG Cailin. Progress in Functions, Allelic Variations and Interactions of Soluble Starch Synthases Genes SSⅡa and SSⅢa in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(3): 227-236. |
| [11] | WANG Yongxiang, YAN Hangang, XU Hancong, FU Yushuang, SHAN Zhuangzhuang, HU Xiaoqing, ZHANG Wenwei, JIANG Ling. Effects of OsESV1 on Starch Synthesis in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(2): 139-149. |
| [12] | LIANG Cheng, XIANG Xunchao, ZHANG Ouling, YOU Hui, XU Liang, CHEN Yongjun. Analyses on Agronomic Traits and Genetic Characteristics of Two New Plant-architecture Lines in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(2): 171-180. |
| [13] | Chunquan ZHU, Qingshan XU, Xiaochuang CAO, Lianfeng ZHU, Yali KONG, Qianyu JIN, Junhua ZHANG. Effects of Substrates with Different Properties on Chilling Tolerance of Early Rice Seedlings [J]. Chinese Journal OF Rice Science, 2021, 35(5): 503-512. |
| [14] | Yujun ZHU, Ziwei ZUO, Zhenhua ZHANG, Yeyang FAN. A New Approach for Fine-mapping and Map-based Cloning of Minor-Effect QTL in Rice [J]. Chinese Journal OF Rice Science, 2021, 35(4): 407-414. |
| [15] | Zheng TIAN, Chunfang ZHAO, Yadong ZHANG, Qingyong ZHAO, Zhen ZHU, Ling ZHAO, Tao CHEN, Shu YAO, Lihui ZHOU, Wenhua LIANG, Kai LU, Cailin WANG, Hongsheng ZHANG. Differences in Eating and Cooking Quality Traits of Semi-waxy japonica Rice Cultivars in Jiangsu Province [J]. Chinese Journal OF Rice Science, 2021, 35(3): 249-258. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||